Health
FDA Approves Zepbound as First Medication for Moderate to Severe Obstructive Sleep Apnea in Adults with Obesity
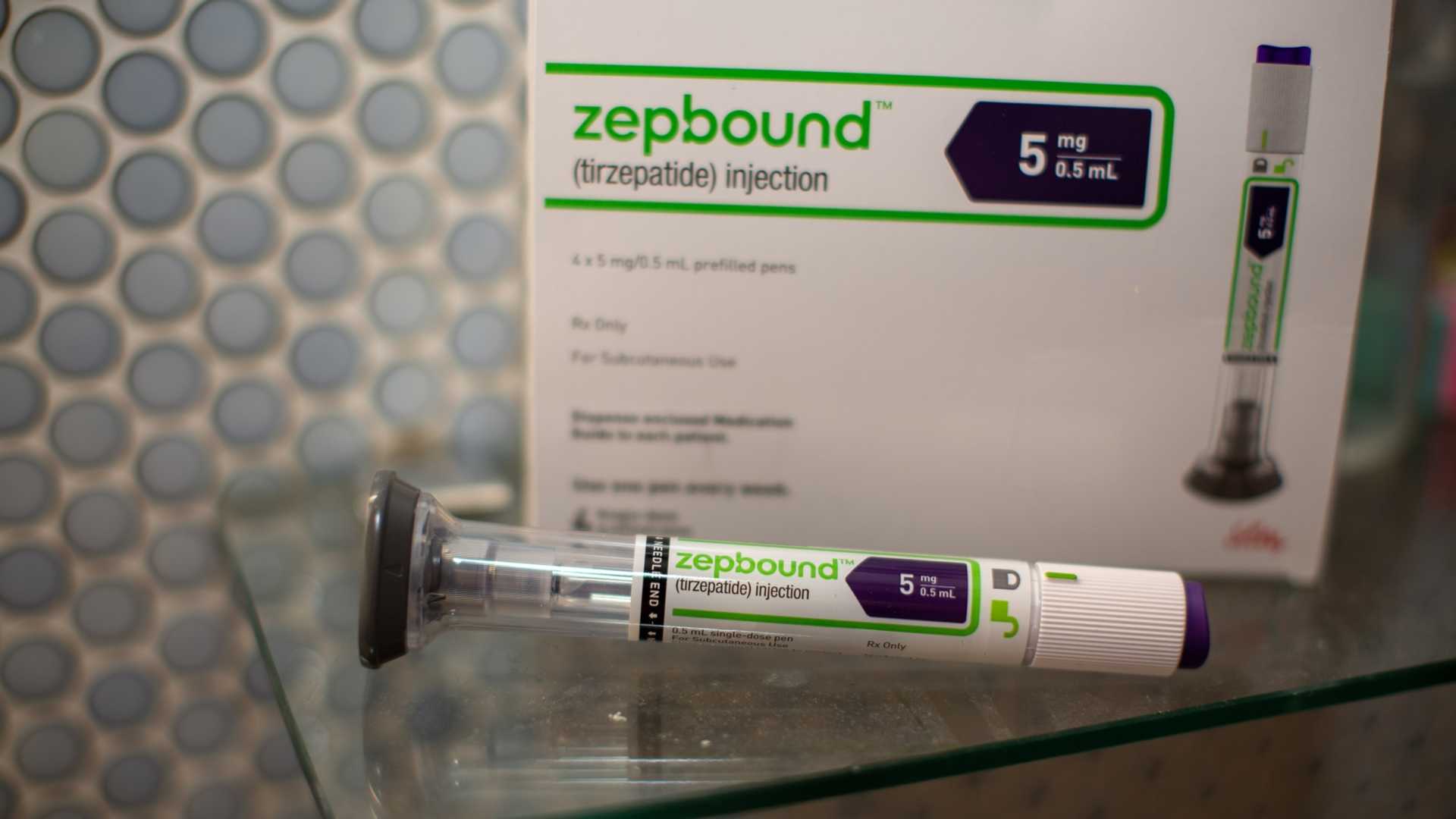
The U.S. Food and Drug Administration (FDA) has approved Zepbound (tirzepatide) as the first medication for the treatment of moderate to severe obstructive sleep apnea (OSA) in adults with obesity. This approval marks a significant step forward in managing a condition that affects millions of Americans, particularly those who are overweight or obese.
Obstructive sleep apnea occurs when the upper airway becomes blocked during sleep, causing pauses in breathing and potentially leading to fatigue, excessive daytime sleepiness, and disrupted sleep. Zepbound works by activating receptors of hormones secreted from the intestine, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which reduce appetite and food intake, thereby leading to weight loss.
The approval is based on results from the SURMOUNT-OSA phase 3 clinical trials, which evaluated Zepbound in adults with moderate-to-severe OSA and obesity, with and without positive airway pressure (PAP) therapy. Participants who received Zepbound experienced a statistically significant and clinically meaningful reduction in events of apnea or hypopnea as measured by the apnea-hypopnea index (AHI) compared to placebo.
After one year of treatment, up to 50% of adults taking Zepbound no longer had symptoms associated with OSA, and those on PAP therapy experienced even better outcomes. The primary measure of efficacy was the change from baseline in AHI at week 52, showing that Zepbound significantly improved breathing disruptions during sleep.
While Zepbound can cause side effects such as nausea, diarrhea, vomiting, and injection site reactions, it is a crucial advancement for patients with OSA who are unable or unwilling to use PAP therapy. The FDA granted Zepbound Fast Track, Priority Review, and Breakthrough Therapy designations for this indication.