Health
IO Biotech’s Phase 3 Results Show Potential in Advanced Melanoma Treatment
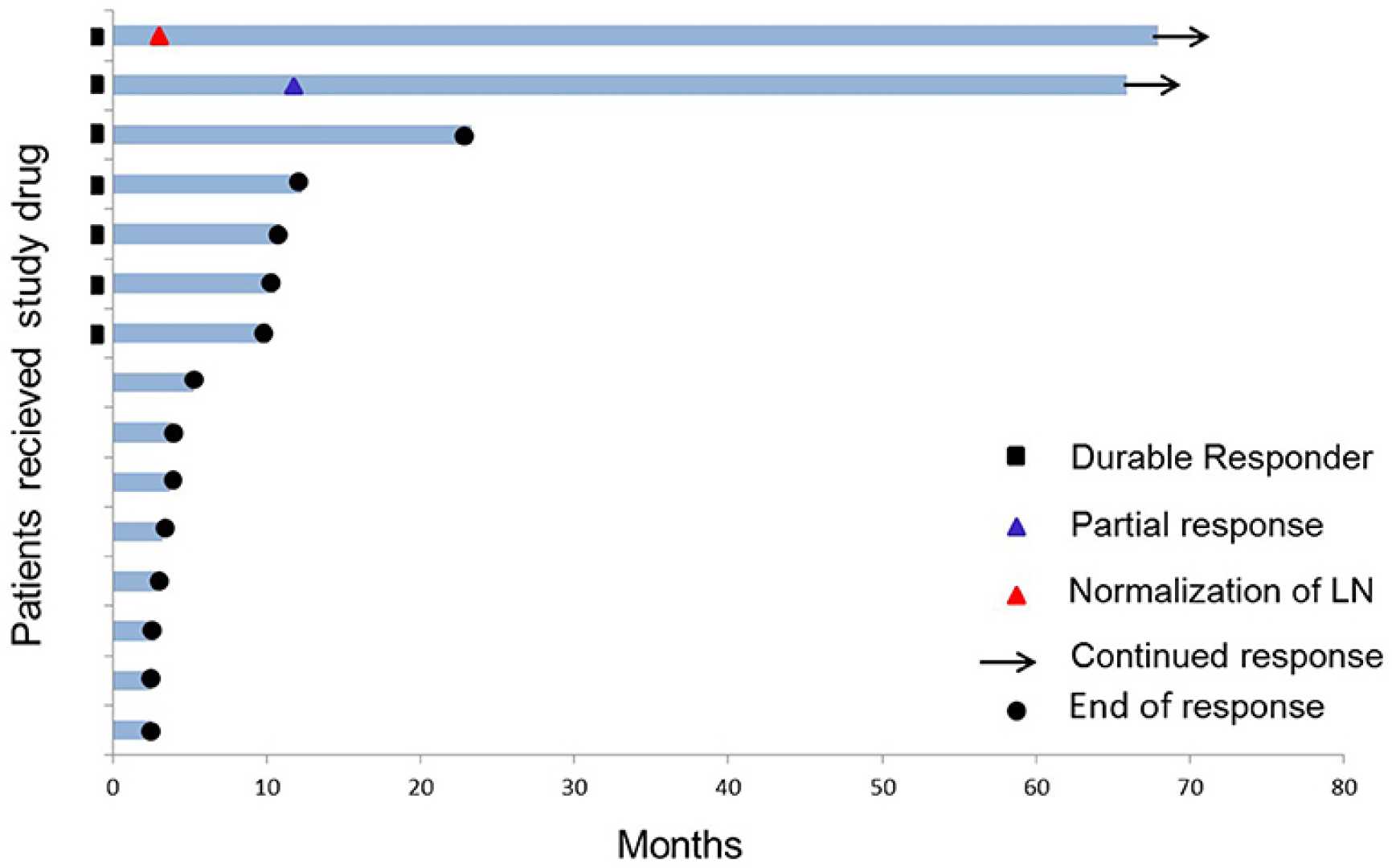
NEW YORK, Aug. 11, 2025 (GLOBE NEWSWIRE) — IO Biotech (NASDAQ: IOBT) announced promising topline results from its pivotal Phase 3 trial of Cylembio combined with Merck‘s KEYTRUDA (pembrolizumab) for treating advanced melanoma. The trial showed a median progression-free survival of 19.4 months for patients receiving both treatments, compared to 11.0 months for those taking KEYTRUDA alone.
While the results were encouraging, they narrowly missed achieving statistical significance at p=0.056. Nonetheless, this trial involved 407 patients across more than 100 sites globally, illustrating a significant effort in evaluating potential treatments for unresectable or metastatic melanoma.
“In this study, we observed a highly encouraging improvement in progression-free survival,” said Dr. Mai-Britt Zocca, CEO of IO Biotech. “The magnitude and durability of clinical effect observed consistently across subgroups supports our confidence in Cylembio’s potential.”
Notably, patients in the trial with PD-L1 negative tumors showed particularly improved results, achieving a median progression-free survival of 16.6 months compared to just 3.0 months for those treated with pembrolizumab alone (HR: 0.54, p=0.006).
Despite the absence of new safety signals, side effects were documented, with 56% of patients experiencing injection site reactions, which were transient and resolved. IO Biotech plans to meet with the FDA this fall to discuss these results and the possibility of submitting a Biologics License Application.
Dr. Omid Hamid, director of clinical research at The Angeles Clinic and Research Institute, emphasized the potential of Cylembio to become a new standard of care in advanced melanoma treatments, especially given its favorable safety profile. “These data show the potential of a therapeutic cancer vaccine in metastatic melanoma,” he said.












