New Study Details Energy Costs of Lunar Rocket Fuel Production
WASHINGTON, D.C. — A new study published by the Proceedings of the National Academy of Sciences (PNAS) outlines the energy requirements for producing rocket fuel on the Moon, presenting insights crucial for future lunar exploration and broader Solar System travel.
As humanity seeks to expand beyond Earth, establishing a fuel production facility on the Moon could provide significant advantages over low-Earth orbit. While launching rockets from Earth to low-Earth orbit consumes significant energy, sending rockets from the Moon could be more efficient, requiring less propellant for deliveries to strategic points like the Earth-Moon Lagrange Point 1.
The research indicates that launching a rocket from Earth requires nearly 25 kg of propellant to transport a single kilogram of payload. In contrast, a rocket launched from the Moon needs just 4 kg of propellant for the same payload. This fundamental shift could enhance the feasibility of further space exploration.
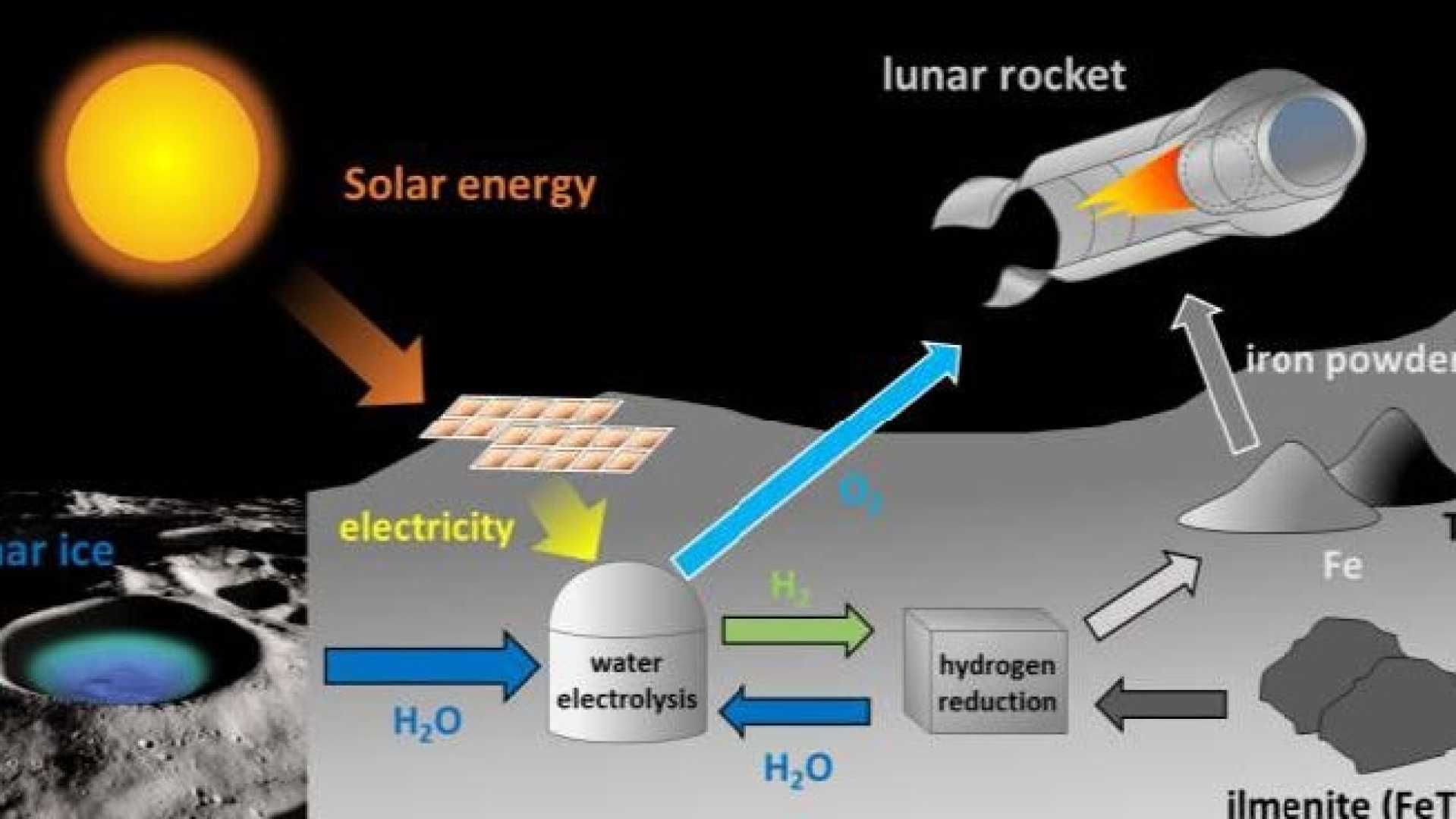
Critical to the fuel production process is the presence of water on the Moon, which can be split into hydrogen and oxygen. Although the prospect of using lunar water is appealing, scientists still need detailed data on the quantity and distribution of water resources on the Moon’s surface.
Pioneering research on Moon regolith—lunar dust composed of a variety of minerals—has shown that it is abundant in oxygen-containing minerals. Notably, a mineral called ilmenite (FeTiO3) has emerged as a potential source for oxygen extraction. While the extraction process is not straightforward, significant advancements have been made in the chemistry needed for isolating oxygen from ilmenite.
The study highlights three major energy-consuming steps in the process: a high-temperature reaction with hydrogen, water splitting for hydrogen regeneration, and converting oxygen to its liquid form. Overall, producing liquid oxygen from ilmenite could require approximately 24 kWh of energy per kilogram.
Optimizing efficiency in these steps could dramatically influence energy requirements. For instance, improving ilmenite separation would reduce the energy cost associated with heating contaminants during processing. The study also notes that concentrated solar power could potentially replace electrical heating, though this was not deeply analyzed.
The researchers emphasize the need to identify viable locations on the Moon with sufficient ilmenite concentrations for harvesting. Their maps identify several promising sites on the Moon’s near side that could facilitate easier access.
Despite the seemingly manageable energy requirement of 24 kWh per kilogram of liquid oxygen, the sheer volume of production needed poses challenges. Estimates suggest an empty SpaceX Starship requires 80 tonnes of liquid oxygen for a lunar launch, complicating logistics for establishing a viable lunar fuel depot.
With current technology, utilizing the solar arrays on the International Space Station could yield about four kilograms of oxygen per hour—an unfeasible rate considering it would take over two years to gather enough to fuel a launch. Some researchers propose that nuclear power might offer an alternative, enabling continuous production regardless of lunar day limitations.
This research offers a preliminary look into the energetic implications of lunar-based fuel production, highlighting the significant infrastructure and resources required to enable deeper space exploration. It stands as a foundation for future studies aimed at determining the best practices for lunar refueling initiatives.
