Health
Pediatric Patients with Psoriasis Show Significant Improvement with Apremilast Treatment, Phase 3 Trial Finds
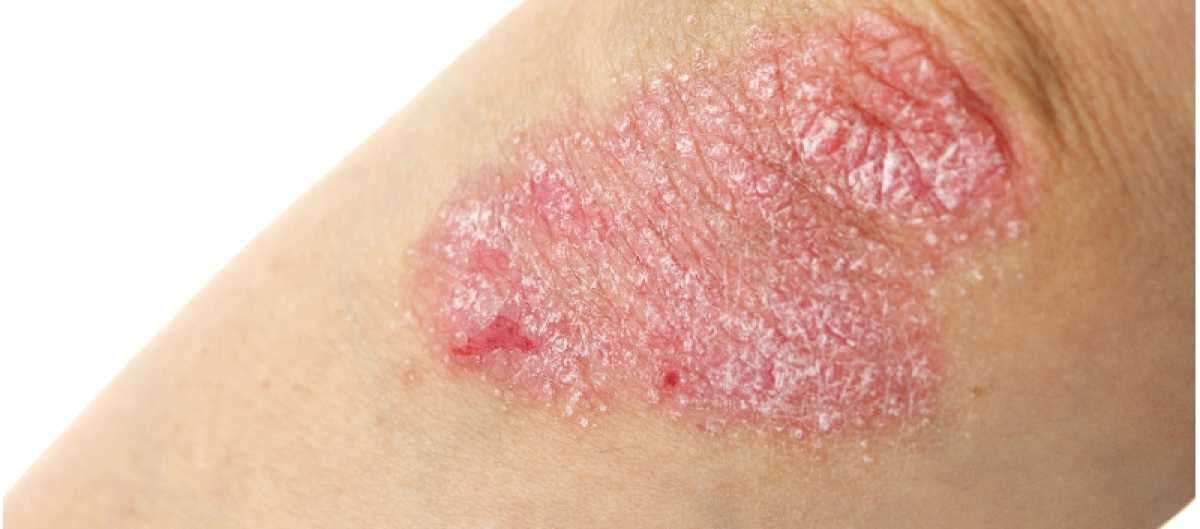
Pediatric patients with psoriasis experienced significant improvement with oral apremilast treatment, according to the results of a phase 3 clinical trial. The study, known as the SPROUT study, aimed to evaluate the efficacy of apremilast in children aged 6 to 17 years with psoriasis.
The SPROUT study was a multicenter, randomized, double-blind, placebo-controlled trial. A total of 245 patients were included in the study and randomly assigned to receive either apremilast or a placebo for 16 weeks.
At the end of the 16-week treatment period, the primary endpoint of a static Physician Global Assessment response was achieved by a significantly higher percentage of patients in the apremilast group compared to the placebo group. Secondary endpoints, such as PASI 75 and PASI 90 responses, also favored the apremilast group.
What makes this study particularly noteworthy is that there are very limited treatment options available specifically for pediatric patients with psoriasis. According to Dr. Loretta Fiorillo, director of pediatric dermatology at the University of Alberta and one of the study authors, there is a need for more research and medications suitable for children.
Currently, biologics are the drug of choice for psoriasis treatment in adults. However, many children are hesitant to undergo treatment with injections. Oral medications like apremilast offer a more convenient option for pediatric patients.
The study results showed consistent improvement in all age and weight groups, with younger patients (aged 6 to 11 years) exhibiting a greater response to apremilast. Parameters including PASI score, itch severity, and scalp psoriasis were significantly improved in the apremilast group.
Fiorillo emphasized that apremilast is a favorable option for children as it only requires taking a pill twice a day and does not involve regular bloodwork. However, adverse events were reported in both the apremilast and placebo groups, with gastrointestinal issues being the most common in the apremilast group.
The findings of the SPROUT study provide valuable evidence for the effectiveness of oral apremilast treatment in pediatric patients with psoriasis. Further research and studies are needed to explore additional treatment options for children.
Proper Nouns: Loretta Fiorillo, University of Alberta, SPROUT study, Healio, Pfizer, Galderma, LEO Pharma, Pierre Fabre












