Business
AbbVie Stock Plummets After Disappointing Schizophrenia Drug Trial Results
AbbVie‘s stock experienced a significant decline on Monday following the release of disappointing results from a phase 2 trial of its schizophrenia treatment drug, emraclidine. The drug failed to meet its primary endpoint, showing no statistically significant reduction in the Positive and Negative Syndrome Scale (PANSS) compared to a placebo. This news led to a 12% drop in AbbVie’s stock price in late-afternoon trading.
The emraclidine drug was a key component of AbbVie’s acquisition of Cerevel Therapeutics in early August for $8.7 billion. Despite this setback, AbbVie remains committed to developing treatments for psychiatric and neurological disorders. The company’s chief scientific officer, Roopal Thakkar, emphasized their ongoing dedication to this area of research.
While the failure of emraclidine is a significant blow, AbbVie’s acquisition of Cerevel Therapeutics also included other promising pipeline programs. Notably, the company’s Parkinson's disease treatment, tavapadon, has shown positive results in recent late-stage trials, offering some optimism for AbbVie’s future pipeline.
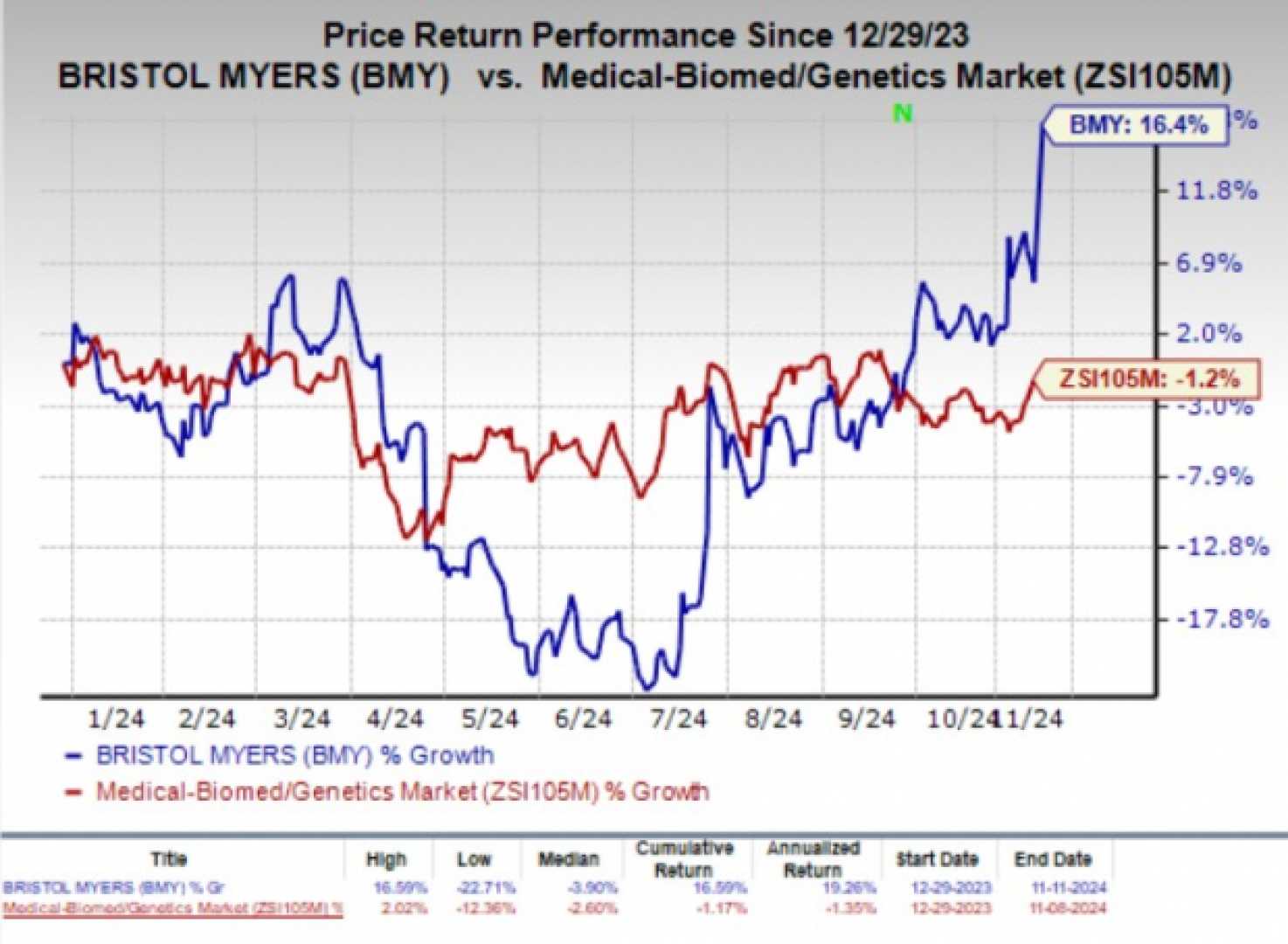
The impact of AbbVie’s news was also felt by its competitors, with Bristol Myers Squibb‘s stock rising more than 11% in afternoon trading on Monday, likely due to the relative strength of its own pipeline compared to AbbVie’s recent setback.
Despite the current dip, analysts suggest that AbbVie’s overall financial health and strong cash flow position could support the stock in the long term. The company’s accrual ratio and free cash flow indicate that its earnings may be understated, and it maintains a strong dividend yield, which could act as valuation support.












