Business
Rapport Therapeutics to Announce RAP-219 Trial Results This September
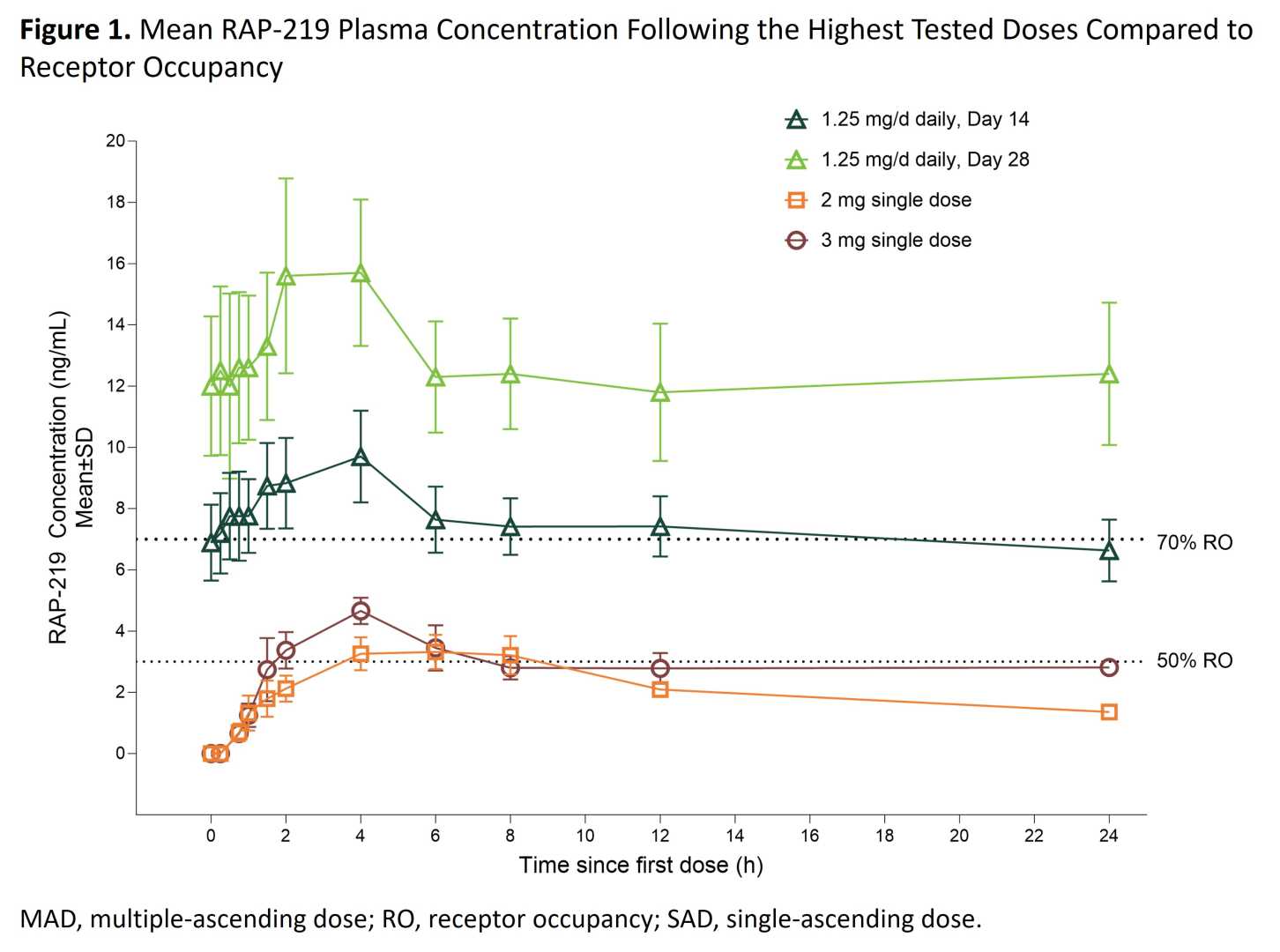
BOSTON and SAN DIEGO, Sept. 05, 2025 (GLOBE NEWSWIRE) — Rapport Therapeutics, Inc. (Nasdaq: RAPP) will host a conference call and live webcast on September 8, 2025, at 8:00 am ET to present topline results from its Phase 2a trial of RAP-219, targeting drug-resistant focal onset seizures.
The clinical-stage biotechnology company focuses on developing small molecule precision medicines for neurological and psychiatric disorders. This trial is significant as it investigates RAP-219’s efficacy in patients who do not respond to standard treatments.
Individuals can join the live conference call through a webcast link or by calling (800) 715-9871 in the U.S. or (646) 307-1963 from other locations. Participants should use conference ID 4762775. A replay will be available on the company’s website for 90 days after the call.
Rapport’s innovative approach is grounded in research related to receptor associated proteins (RAPs) in the brain. This technology platform may enable new treatments for various neurological conditions.
Alongside RAP-219, the company is also exploring therapies for bipolar mania and diabetic peripheral neuropathic pain, with additional programs addressing chronic pain and hearing disorders.












