Health
STRATA Skin Sciences Expands Coverage for XTRAC Laser Treatment
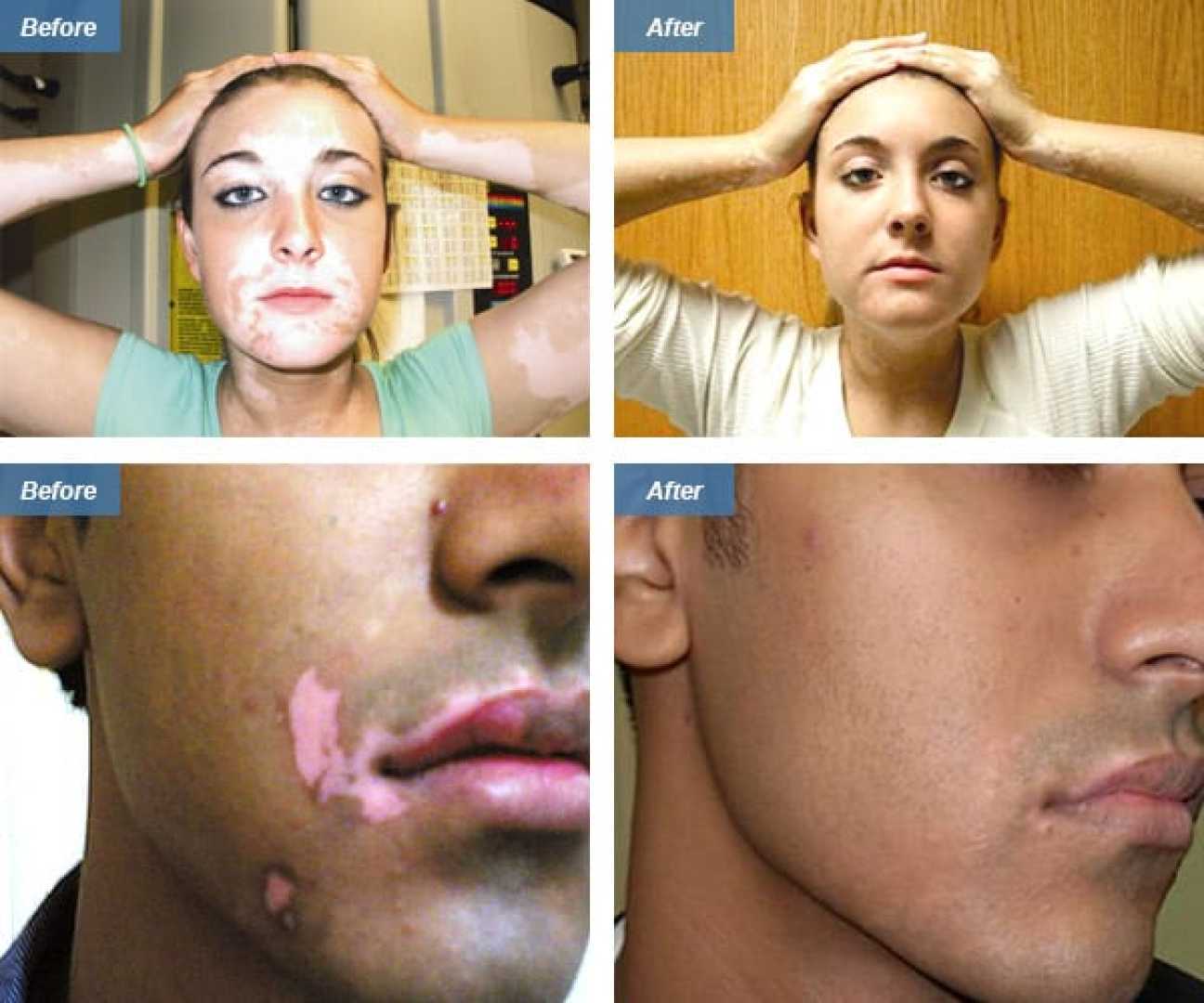
HORSHAM, Pa., Aug. 07, 2025 (GLOBE NEWSWIRE) — STRATA Skin Sciences, Inc. announced plans to work with the Centers for Medicare & Medicaid Services (CMS) to expedite temporary codes for its XTRAC® excimer laser treatment. The American Medical Association‘s CPT Editorial Panel recently approved updated codes that will allow reimbursement for XTRAC treatments for various inflammatory and autoimmune skin conditions, including vitiligo and atopic dermatitis, starting January 1, 2027.
The new codes could significantly broaden reimbursement options, potentially tripling the patient population to over 30 million. STRATA is seeking temporary CMS codes to allow these treatments to be reimbursable as early as 2026. “Expanding access to XTRAC® is not just about increasing device usage; it’s about providing effective treatment for millions suffering from chronic skin conditions,” said STRATA President and CEO Dr. Dolev Rafaeli.
Recent peer-reviewed studies have reinforced the effectiveness of excimer lasers in treating vitiligo. One study showed a 100% response rate and 96% pigment stability among patients using the laser in combination with JAK inhibitors, promising results that support the emergence of XTRAC as a drug-free treatment alternative.
On the legal front, Dr. Rafaeli updated stakeholders regarding an ongoing lawsuit against LaserOptek America. The Federal District Court has permitted STRATA to add LaserOptek Co. Ltd as a defendant, reinforcing the company’s position on misleading advertising claims. STRATA originally filed the lawsuit in August 2024 over alleged false statements made regarding the marketing of Pallas lasers.
STRATA’s innovative approach to treatment and legal strategies position it uniquely as it continues to advance excimer laser therapies and contend with market challenges.












