Health
Stem Cell Trials Offer New Hope for Parkinson’s Disease Patients
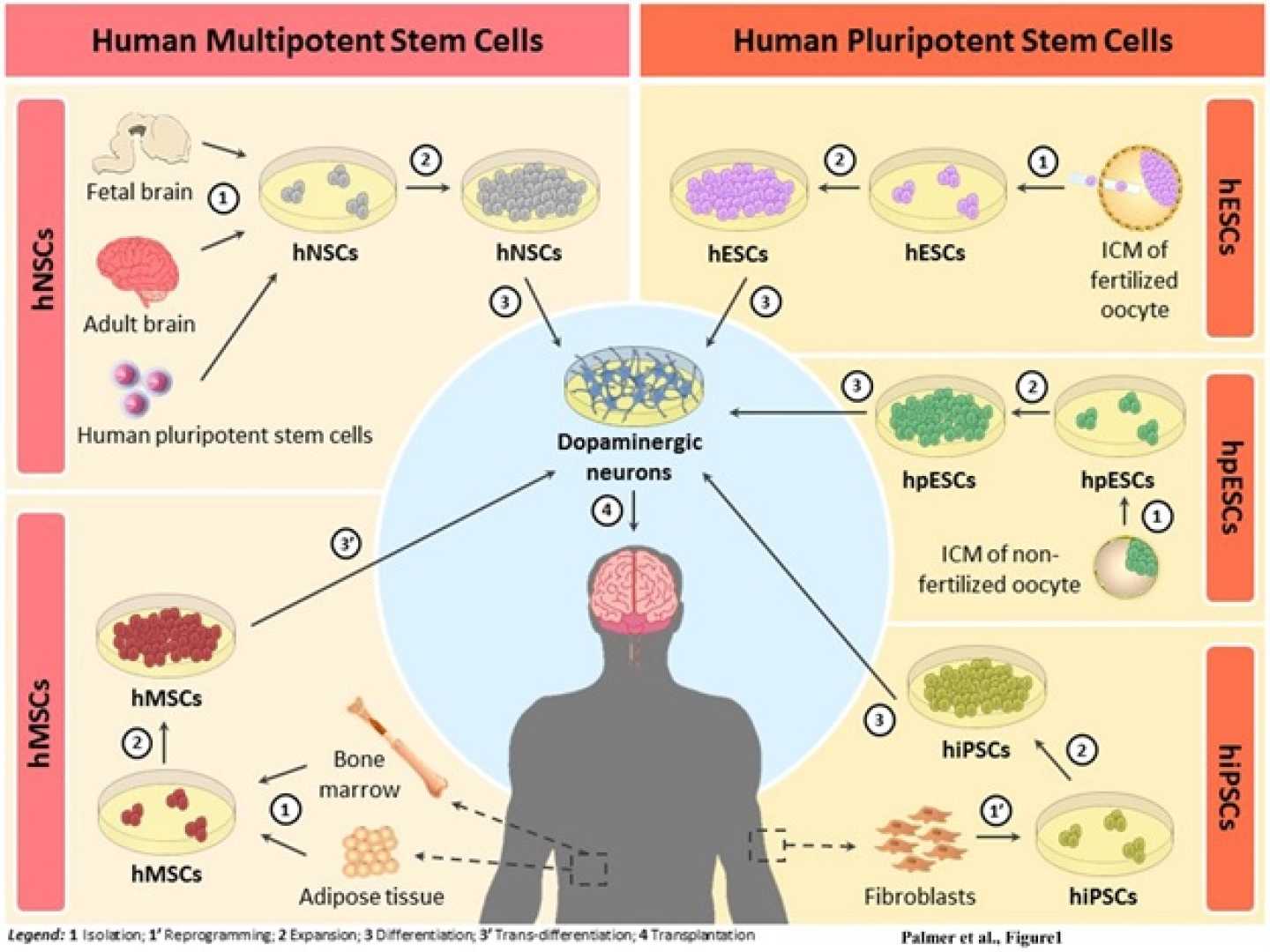
CAMBRIDGE, Mass. — Two small clinical trials have rekindled hope in the fight against Parkinson’s disease by exploring the possibility of using stem cells to replace damaged nerve cells in the brain. Published April 16, 2025, in the journal Nature, the studies suggest that cell injections can safely introduce dopamine-producing neurons that are typically lost in Parkinson’s.
These trials involved 19 patients, 12 in a study led by Viviane Tabar at Memorial Sloan Kettering Cancer Center in New York City, and seven patients in a separate study rooted in Japan. Researchers injected cells derived from stem cells into the patients’ brains in an effort to restore dopamine levels and alleviate motor symptoms associated with the disease, which affects more than 8 million individuals globally.
“These results mark an encouraging first step in stem cell-based therapy for Parkinson’s disease,” said Ole Isacson, a neurologist affiliated with Harvard Medical School and McLean Hospital. He noted that previous attempts to transplant fetal brain tissue to treat Parkinson’s were marred by ethical challenges and safety issues, including severe side effects for some participants.
In both recent clinical trials, the focus was on safety rather than efficacy, a consensus shared by the lead researchers. No serious side effects arose from the stem cell injections, and the study participants did not suffer any of the severe complications often associated with earlier fetal tissue transplants.
Jun Takahashi, a coauthor and neurosurgeon at Kyoto University, emphasized that the findings affirm the safety of these stem cell therapies. “The main conclusion is that we confirmed the safety of these kinds of cells,” Takahashi stated. Participants experienced mild adverse events linked to immunosuppressive drugs, necessary when introducing foreign cells, but there were no tumor formations or uncontrolled cell growth, which have been concerns in similar treatments.
The studies provide early indications that the stem cell-derived cells may help manage Parkinson’s symptoms; however, the small sample sizes limit the conclusions that can be drawn. Takahashi and colleagues noted some patients exhibited signs of dopamine production and reported minor improvements in symptoms, although definitive efficacy remains to be established.
Tabar highlighted that larger multicenter trials will be necessary to comprehensively evaluate the treatment’s effects. “We need multicenter, large-sample trials with multiple controls,” she remarked. Plans for a larger study involving approximately 100 participants using the same cells are expected to launch later this year.
Despite the initial promising data, both Tabar and Takahashi agree that further research is essential. The ongoing development of these studies aims to assess the effectiveness of the injected cells over a longer duration and to refine the approach to treatment.
The studies not only provide hope for Parkinson’s patients but also pave the way for future research into stem cell applications in neurodegenerative diseases. The scientific community continues to watch these developments closely, expecting that subsequent trials will offer deeper insights and potentially transformative therapies for afflictions that significantly impair quality of life.












