Health
Experimental mRNA Vaccine Shows Promise Against Deadly Pancreatic Cancer
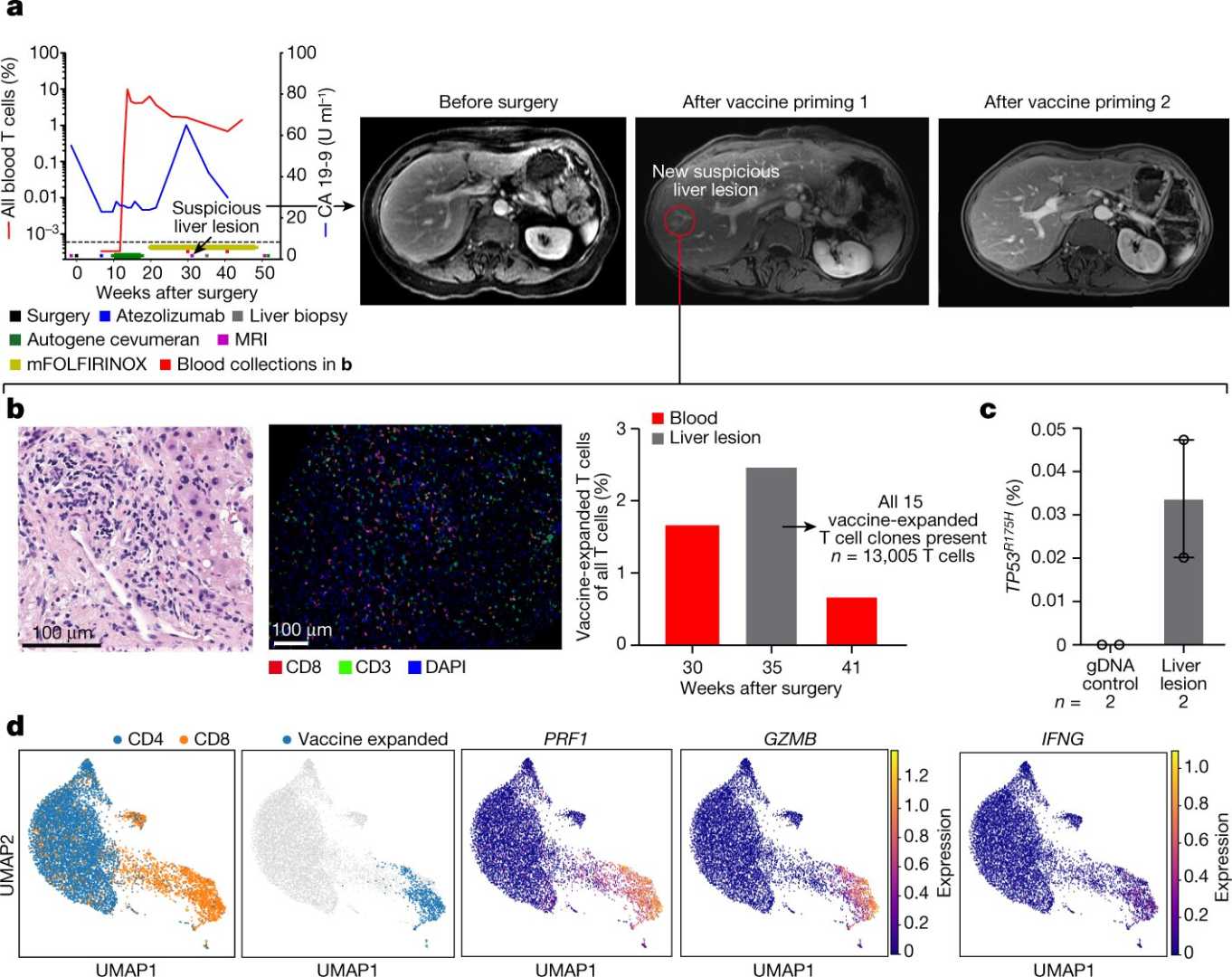
New York, NY — A pioneering mRNA-based vaccine targeting pancreatic cancer has shown hope in a recent phase 1 clinical trial conducted by researchers at Memorial Sloan Kettering Cancer Center (MSK). This vaccine, which aims to enhance the immune system’s ability to combat cancer, proved successful in generating a lasting immune response and reducing the risk of cancer recurrence among participants after surgery.
Results published in the journal Nature indicate that in a small group of 16 patients, half showed a robust immune response that persisted for almost four years following treatment. Those with an active immune response were significantly more likely to remain free of cancer three years post-surgery.
“With RNA vaccine technology, we can teach the immune system to recognize pancreatic cancer cells,” said Dr. Vinod Balachandran, the principal investigator of the trial. “The immune response could potentially last for many years. The ability to trigger a robust, long-lasting immune response is essential for any cancer vaccine.”
Pancreatic cancer remains one of the most challenging cancers to treat, significantly due to late-stage diagnoses. According to MSK and the American Cancer Society, only 13% of patients survive five years after diagnosis, highlighting the urgent need for innovative therapies. Traditional treatments such as chemotherapy and radiation have proven largely ineffective against this malignancy.
“We were surprised that the immune system responded to this approach, which demonstrates potential in treating such a difficult cancer,” Balachandran added. “Testing our vaccine in this setting could lead to impactful treatment options for patients.”
The phase 1 trial involved personalized cancer vaccines tailored to each patient’s unique cancer proteins. Post-surgery, participants also received atezolizumab, a monoclonal antibody, alongside standard chemotherapy.
While the initial results are promising, Balachandran cautioned that further research is still needed. MSK is planning a larger study to evaluate the efficacy of these therapeutic vaccines on over 250 patients with surgically removable pancreatic tumors.
“In the future, if this method works for pancreatic cancer, it might be applicable for other types of cancer as well,” Balachandran stated.
As researchers forge ahead with this innovative treatment, the hopeful findings are a beacon for pancreatic cancer patients and underscore the critical need for ongoing research and investment in cancer vaccine technology.












