Health
FDA Approves Over-the-Counter Multiplex Tests for Respiratory Viruses
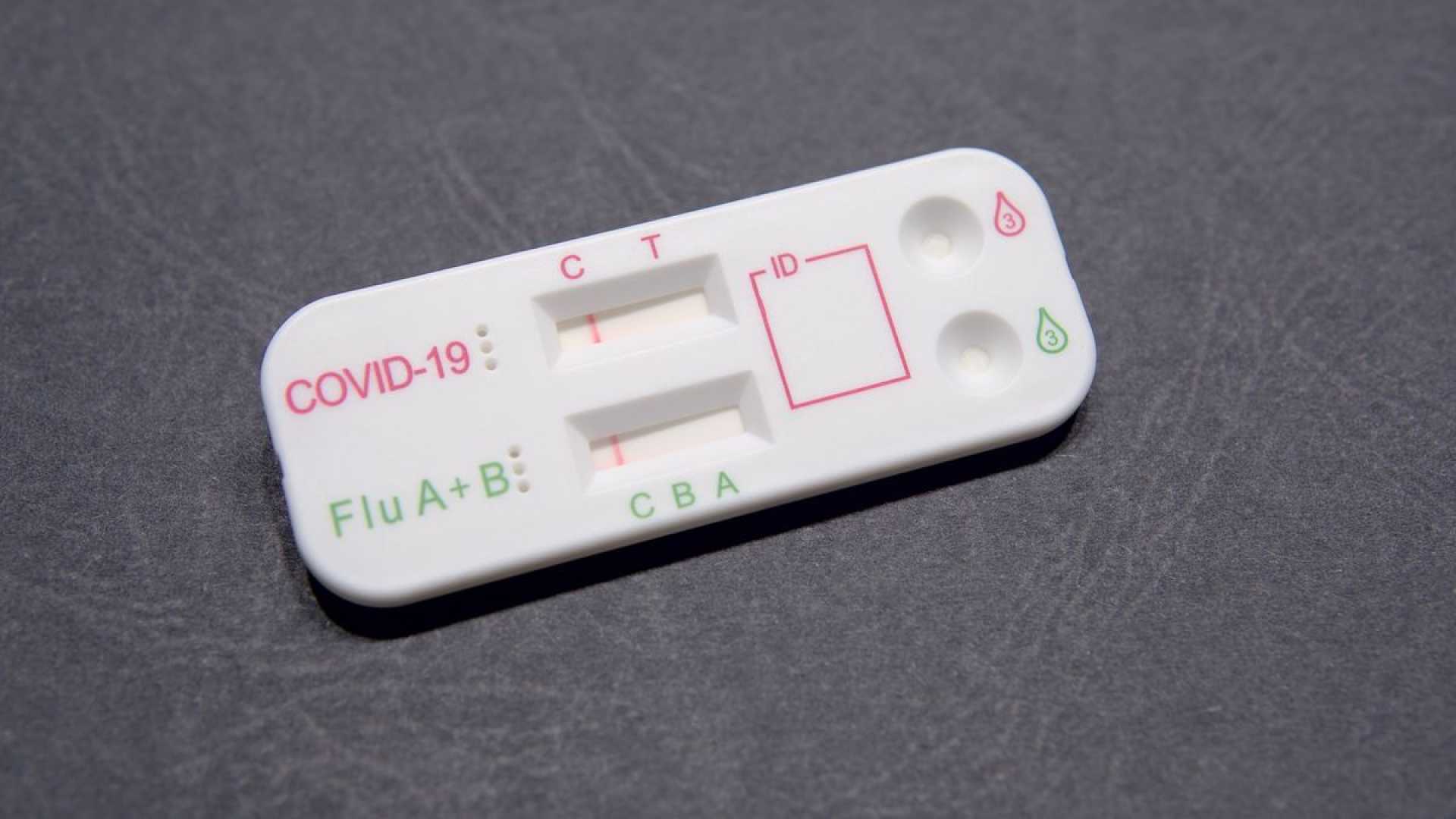
With the onset of the respiratory virus season, a new diagnostic tool is set to become widely available. Over-the-counter multiplex tests capable of detecting both influenza and COVID-19 in a single sample are now hitting pharmacy shelves across the United States.
These combination tests offer a means to rapidly determine the cause of respiratory symptoms, which often overlap and make it difficult to discern between various viruses. The tests have received emergency use authorization from the Food and Drug Administration (FDA), thanks in part to the efforts facilitated by the National Institutes of Health (NIH) under its Rapid Acceleration of Diagnostics (RADx) initiative.
Julie Sullivan, the Chief Operating Officer of Emory University’s RADx Center, stated, “These tests are significant in helping to quickly distinguish between influenza A, influenza B, and COVID-19, enabling faster and more accurate care plans.” The Atlanta Center for Microsystems Engineered Point-of-Care Technologies (ACME POCT) at Emory University played a crucial role in testing and evaluating these diagnostics.
Historically, determining the specific virus responsible for respiratory illness required a visit to a healthcare facility and a lengthier laboratory process. These multiplex tests simplify and expedite the testing process, employing a nasal swab to collect samples. Users simply follow detailed instructions to obtain results, usually within 10 to 30 minutes.
While these tests offer convenience and speed, experts caution that like all rapid tests, they have limitations. Antigen tests used in these kits are generally less sensitive than polymerase chain reaction (PCR) tests performed in laboratories, meaning that they might miss an infection if the viral load is low.
However, the ability to perform these tests at home significantly aids early detection and helps in starting antiviral treatments at the opportune time. Dr. Benjamin S. Arnold, a principal investigator at the ACME/RADx initiative, emphasized the importance of early testing, stating, “Antiviral treatments for both COVID-19 and flu are most effective when initiated early.”
Concerns remain around the correct interpretation of test results, especially since symptoms of various infections can be similar. It is recommended that if someone tests negative but exhibits symptoms, they should take another test 48 hours later to confirm the result.
These tests are widely available at pharmacies such as CVS and Walgreens, as well as through online retailers like Amazon. Prices typically range from $10 to $12 per test, making them accessible to many. People with health savings accounts or flexible spending accounts may use these for reimbursement.
Overall, while the introduction of these combination tests marks a significant advancement in public health capability, users are advised to follow instructions meticulously and consult healthcare providers for guidance, particularly if they belong to at-risk groups.












