Health
RSV Vaccines Prove Highly Effective, But Uptake Remains Low Among At-Risk Populations
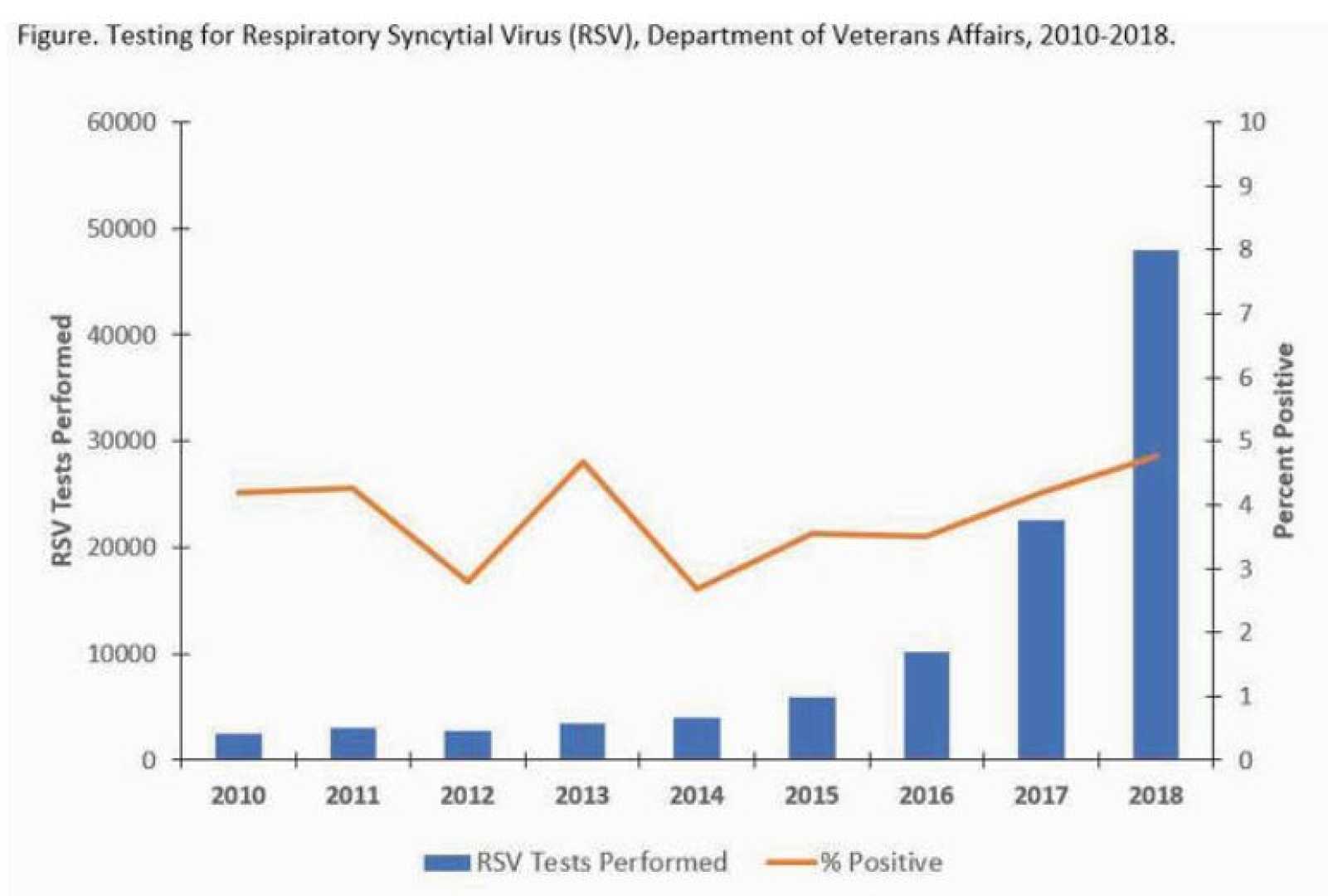
Recent research published in *The Lancet* highlights the significant effectiveness of respiratory syncytial virus (RSV) vaccines, particularly among older adults and those with underlying health conditions. The vaccines, which include RSVPreF3 (Arexvy; GSK), RSVpreF (Abrysvo; Pfizer), and mRNA-1345 (mRESVIA; Moderna), were approved by the FDA in 2023 and have shown an 80% effectiveness in preventing hospitalization, ICU admission, and death among adults aged 60 years and older.
The study, which utilized data from a large electronic health record network including the CDC and various US healthcare networks, found that despite the vaccines’ efficacy, the uptake of RSV vaccination during the 2023-2024 winter season was disappointingly low. Only 24% of adults aged 60 years and older received an RSV vaccination, compared to 50% who received an influenza vaccine for the same population.
Experts emphasize the critical need for increased vaccination rates, especially among high-risk groups. “The evidence is clear; individuals should get vaccinated if they have conditions that place them at risk for severe disease. For older adults and those with chronic conditions, RSV should be considered as serious as the flu, and they should get vaccinated,” said Angela Branche, MD, an infectious diseases researcher at the University of Rochester Medical Center (URMC).
The US Advisory Committee on Immunization Practices (ACIP) has updated its guidelines to recommend a single dose of any FDA-approved RSV vaccine for adults 75 years and older and for adults 60 to 75 years who are at increased risk for severe RSV. This move aims to build public confidence in the vaccines and simplify implementation for providers and pharmacies.
Despite the positive clinical data, vaccine manufacturers like Moderna are facing challenges with RSV vaccine sales. Moderna’s RSV vaccine, mRESVIA, saw significantly lower-than-expected sales in the third quarter of 2024, partly due to late FDA approval and limited CDC recommendations for its use.












