Health
Study Reveals Mixed Accuracy in FDA-Authorized COVID-19 Antigen Tests
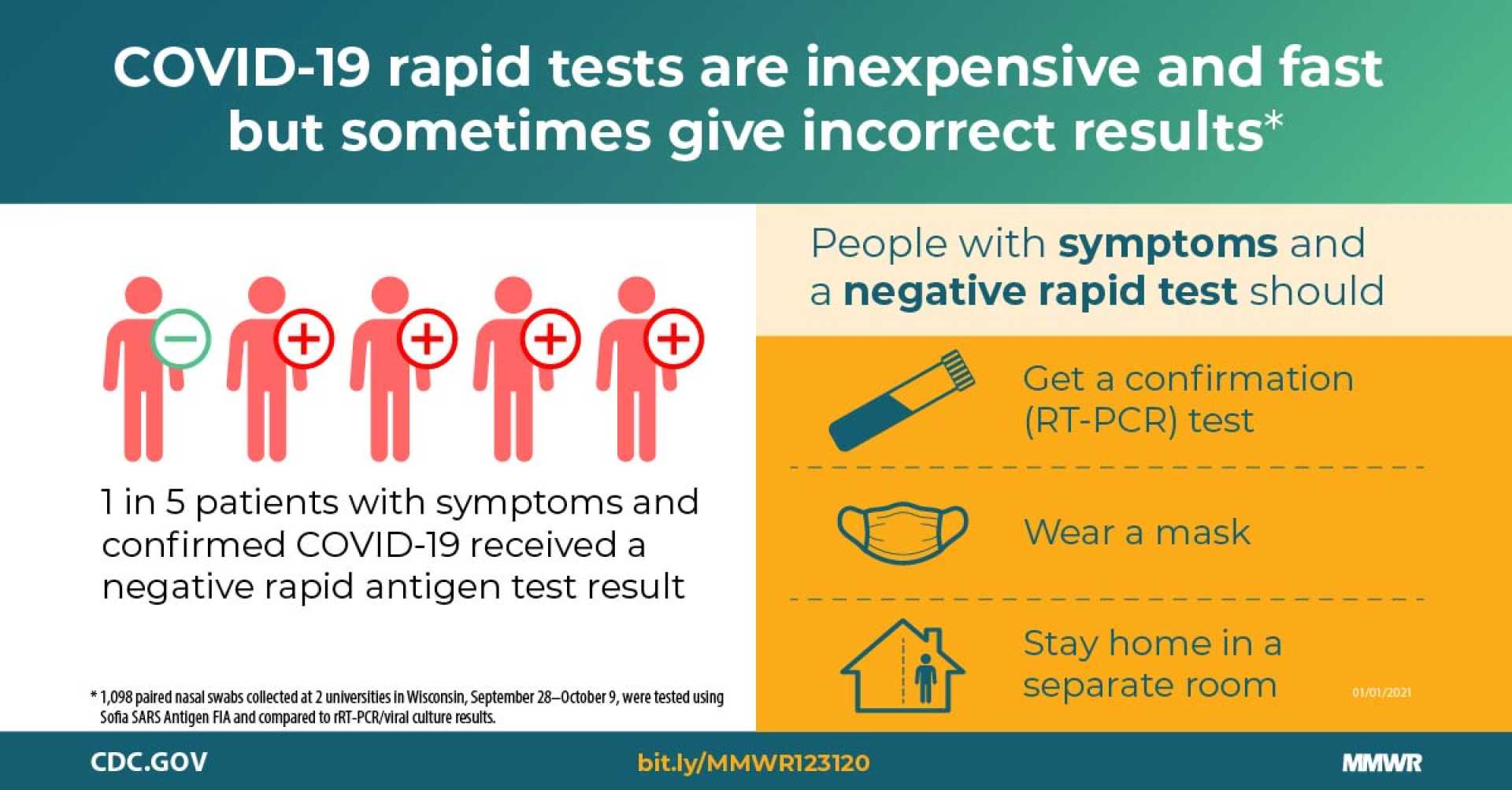
ODENSE, Denmark — A new systematic review published in Clinical Microbiology and Infection investigates the accuracy of FDA-authorized SARS-CoV-2 rapid antigen tests. Researchers from Cochrane Denmark and the Centre for Evidence-Based Medicine Odense assessed data from 13 preapproval and 26 postapproval studies involving over 15,500 individuals.
The study compares test performance before and after receiving Emergency Use Authorization (EUA). Although manufacturers have faced scrutiny over exaggerated claims, the findings revealed that diagnostic accuracy largely aligns with initial manufacturer reports following real-world use. Overall, postapproval studies maintained an average sensitivity of 84.5%, slightly below the preapproval findings of 86.5%.
However, two notable test brands, LumiraDx and SOFIA, exhibited reduced sensitivity postapproval—10.9% and 15.0% lower, respectively. Both had previously reported sensitivity levels exceeding 96%, indicating potential overestimations or issues with newer virus variants affecting their performance.
Out of the 61 FDA-authorized test brands reviewed, only 21% had postapproval studies that adhered to manufacturers’ instructions. The 79% of tests lacking such evaluations raised concerns about ongoing oversight, particularly during public health emergencies when rapid testing became pivotal.
The study highlighted that most postapproval studies were independently conducted and showcased high methodological rigor, indicating no significant bias from industry sponsorship.
Rapid antigen tests have been crucial throughout the COVID-19 pandemic, allowing for swift diagnostics for essential workers and at-home users. The accuracy of these tests influences public health responses, containment measures, and healthcare system readiness. A failure to accurately diagnose can lead to unchecked virus spread or unnecessary isolation.
This research underscores the need for transparent and continuous evaluations of diagnostic tools, especially given the chance of future health emergencies requiring rapid test deployment. While most FDA-authorized tests showed acceptable accuracy, differences in performance among certain brands emphasize the necessity of ongoing monitoring and evaluation.












