Health
FDA Faces Challenges Amid Regulatory Changes and New Drug Approvals
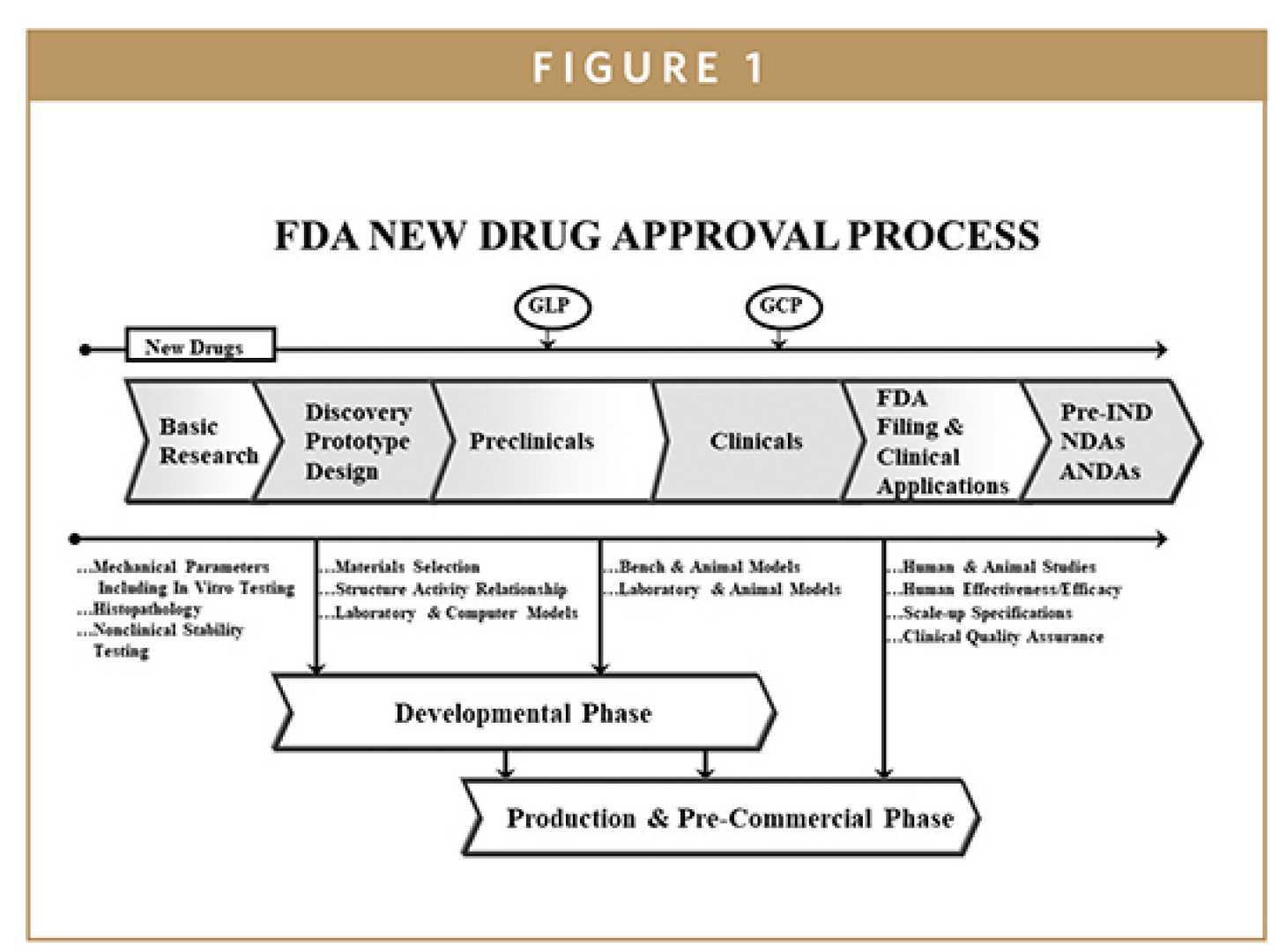
Washington, D.C. — The Food and Drug Administration (FDA) is confronting a turbulent future as it faces a health secretary perceived as hostile to its core mission. This situation raises significant concerns within the healthcare community regarding regulatory processes and public health.
In a positive development, Novartis has secured approval in Switzerland for Coartem Baby, the first medication designed to treat malaria in babies and young children. The new dissolvable, cherry-flavored formulation is expected to improve administration to this vulnerable population, complementing the original Coartem, launched in 1999.
Meanwhile, the Centers for Disease Control and Prevention (CDC) announced the end of its emergency response to the H5N1 bird flu, attributing this decision to a decline in animal infections and the absence of human cases since February. Future updates about the virus will now be incorporated into routine seasonal influenza reports.
In cancer treatment updates, a study published by JAMA Open Network shows that immune checkpoint inhibitors have significantly improved survival rates among cancer patients. The research indicates that privately insured patients have seen a two-year survival rate increase from 29% to 46%, while publicly insured patients’ survival rates improved from 16% to 28%.
FDA Commissioner Marty Makary has vowed to significantly reduce unnecessary animal testing mandates currently required for new drug approval processes. Makary indicated that the agency would explore various approaches to potentially replace or refine these testing requirements.
Moreover, ProKidney is making strides with its renal autologous cell therapy, rilparencel, which is aimed at treating diabetes and chronic kidney disease (CKD). Preliminary results from the phase 2 REGEN-007 clinical trial suggest that patients who received rilparencel demonstrated a 78% improvement in the annual decline of their estimated glomerular filtration rate (eGFR) slopes compared to pre-treatment levels.
CEO Bruce Culleton expressed optimism about the trial results, highlighting plans to present full findings at the upcoming ASN’s 2025 Kidney Week. ProKidney will also hold a Type B meeting with the FDA later this summer to discuss using eGFR slope as a potential surrogate endpoint for accelerated approval of rilparencel.












