Health
Daré Bioscience Reports Positive Results for Ovaprene Contraceptive Trial
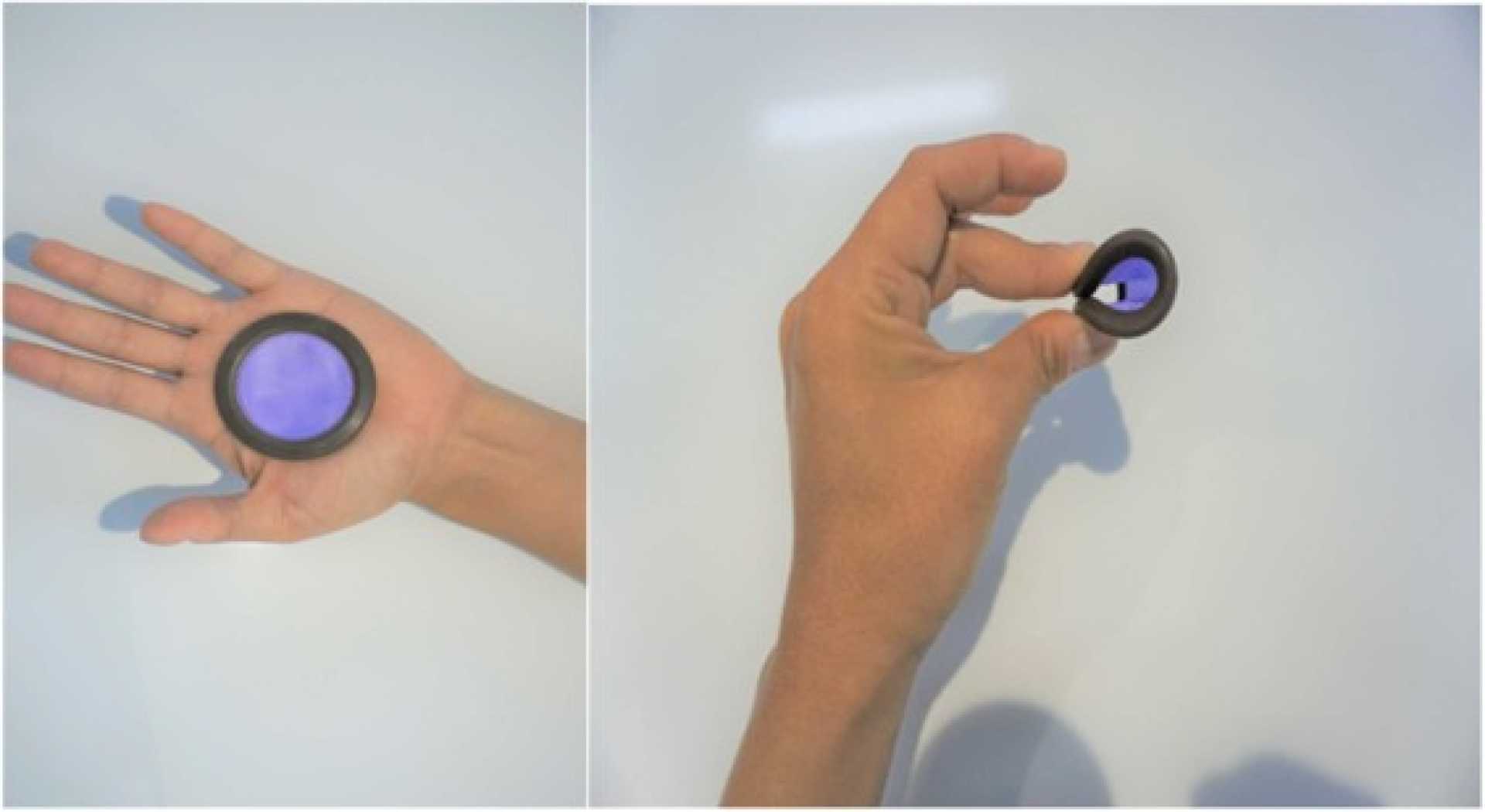
SAN DIEGO, July 14, 2025 (GLOBE NEWSWIRE) — Daré Bioscience, Inc. (NASDAQ: DARE) announced positive interim results from its Phase 3 clinical trial of Ovaprene®, a hormone-free intravaginal contraceptive, today. The trial aims to evaluate the contraceptive effectiveness, safety, and acceptability of the product.
The independent Data Safety Monitoring Board (DSMB) reviewed the interim data and recommended that the trial continue without modifications. Approximately 9% of the participants in the study had experienced a pregnancy, which aligns with the company’s expectations based on earlier tests of Ovaprene.
“We are encouraged by these interim results, which reinforce the potential of our hormone-free contraceptive candidate,” said Sabrina Martucci Johnson, President and CEO of Daré Bioscience. “With millions of women seeking effective, hormone-free birth control, Ovaprene could address significant needs in women’s health.”
No serious safety concerns were noted, although about 17% of the participants discontinued due to vaginal odor, the most reported side effect. Overall, participants indicated they would likely use Ovaprene if available.
The pivotal Phase 3 trial is enrolling around 250 women aged 18-40 across five sites, with an expected duration of 12 months. The primary goal is to ascertain the typical use pregnancy rate over 13 menstrual cycles. Secondary goals include assessing the product’s safety and user acceptability.
Bayer has the right to commercialize Ovaprene in the U.S. after the clinical trial, contingent on a $20 million payment. Daré stands to gain additional milestone payments and royalties based on sales.












