Health
FDA Faces Pressure on Ultraprocessed Foods Regulation
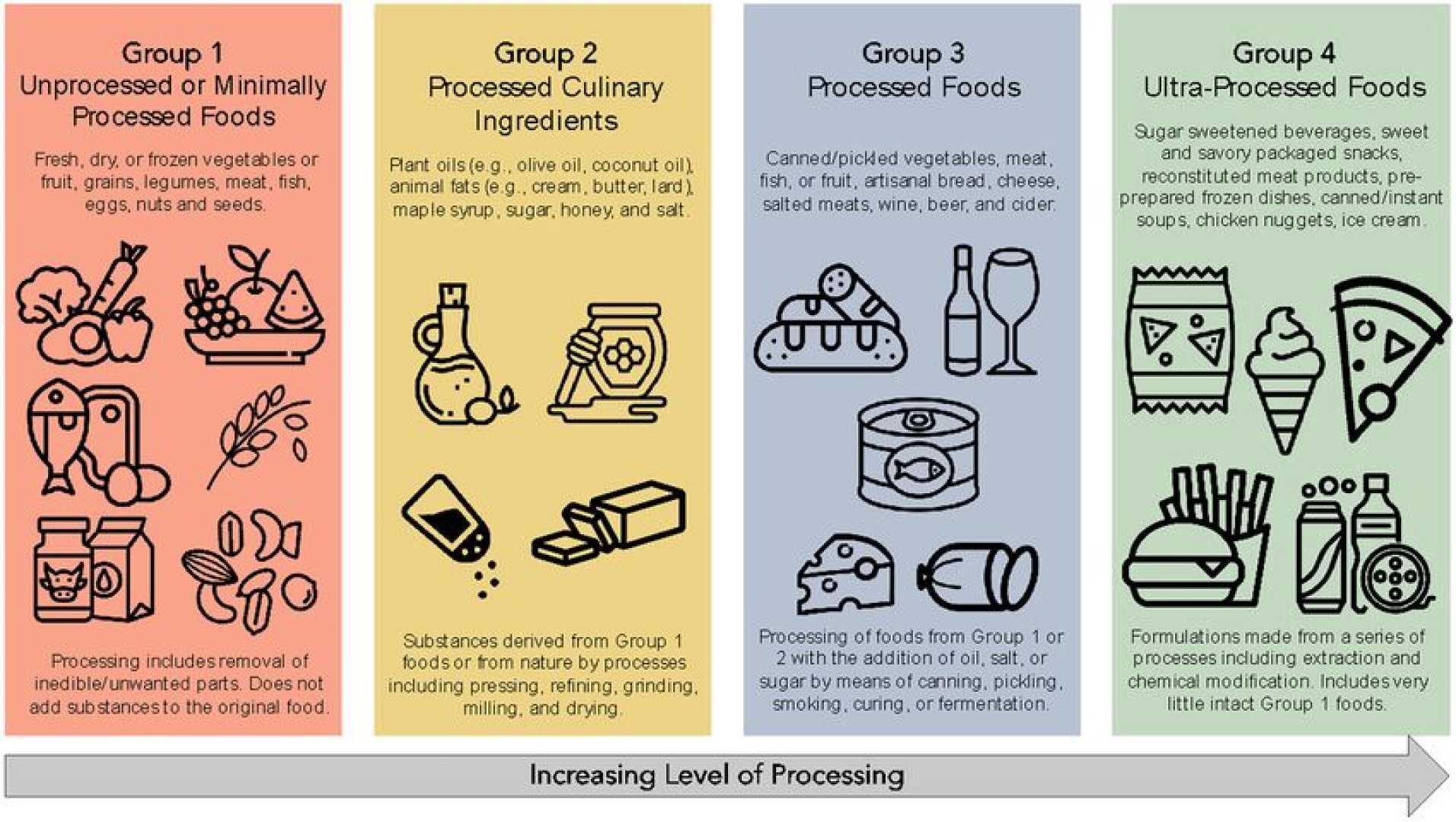
WASHINGTON, D.C. — Federal health officials are seeking input on how to define ultraprocessed foods, paving the way for potentially new regulations. Former FDA Commissioner Dr. David Kessler filed a petition on Wednesday urging that certain ingredients be reclassified as not ‘generally recognized as safe’ (GRAS).
Kessler’s challenge aims to address ingredients like refined sugars and flours used in many ultraprocessed foods. If the FDA accepts his petition, food manufacturers may have to reformulate their products or demonstrate the safety of their ingredients within 12 months.
‘Kessler has given the FDA a way to define the vast majority of ultraprocessed foods,’ Marion Nestle, a nutrition expert from New York University, stated. She emphasized the significance of this move for Health and Human Services Secretary Robert F. Kennedy Jr.’s ‘Make America Healthy Again’ (MAHA) initiative.
Federal health officials have acknowledged the need for more regulations regarding ultraprocessed foods, which have been linked to rising rates of obesity, diabetes, and heart disease. The FDA is required to respond to Kessler’s petition within 180 days, effectively accelerating the timeline for potential regulation.
Kessler, who oversaw the implementation of nutrition labels in the 1990s, argues that finding a legal framework for defining ultraprocessed foods is crucial. ‘The term has resonated with the public, but it’s going to be hard to define legally what’s included,’ he noted in a CNN interview.
The petition highlights several ingredients, including refined flours and syrups, emphasizing that these components contribute to health issues. Nutrition experts like Christopher Gardner warn that additives help make ultraprocessed foods appealing, suggesting a shift away from these ingredients could lead to healthier eating habits.
Experts widely viewed Kessler’s petition as a bold move that could significantly reshape the food industry. ‘This proposal reflects the true meaning of GRAS, which would exclude many products on our grocery shelves,’ Dr. Walter Willett of Harvard stated.
However, proposed changes could face resistance from food manufacturers. Michael Taylor, a former FDA official, noted that past regulatory changes, like the ban on trans fats, ultimately led companies to adapt. ‘The handwriting was on the wall,’ he recalled.
As the FDA responds to Kessler’s petition, the results may influence the upcoming MAHA Commission report, which is set to address chronic illness drivers in children. The report is anticipated to present recommendations soon.












