Health
Apple Introduces New Sleep and Hearing Health Features
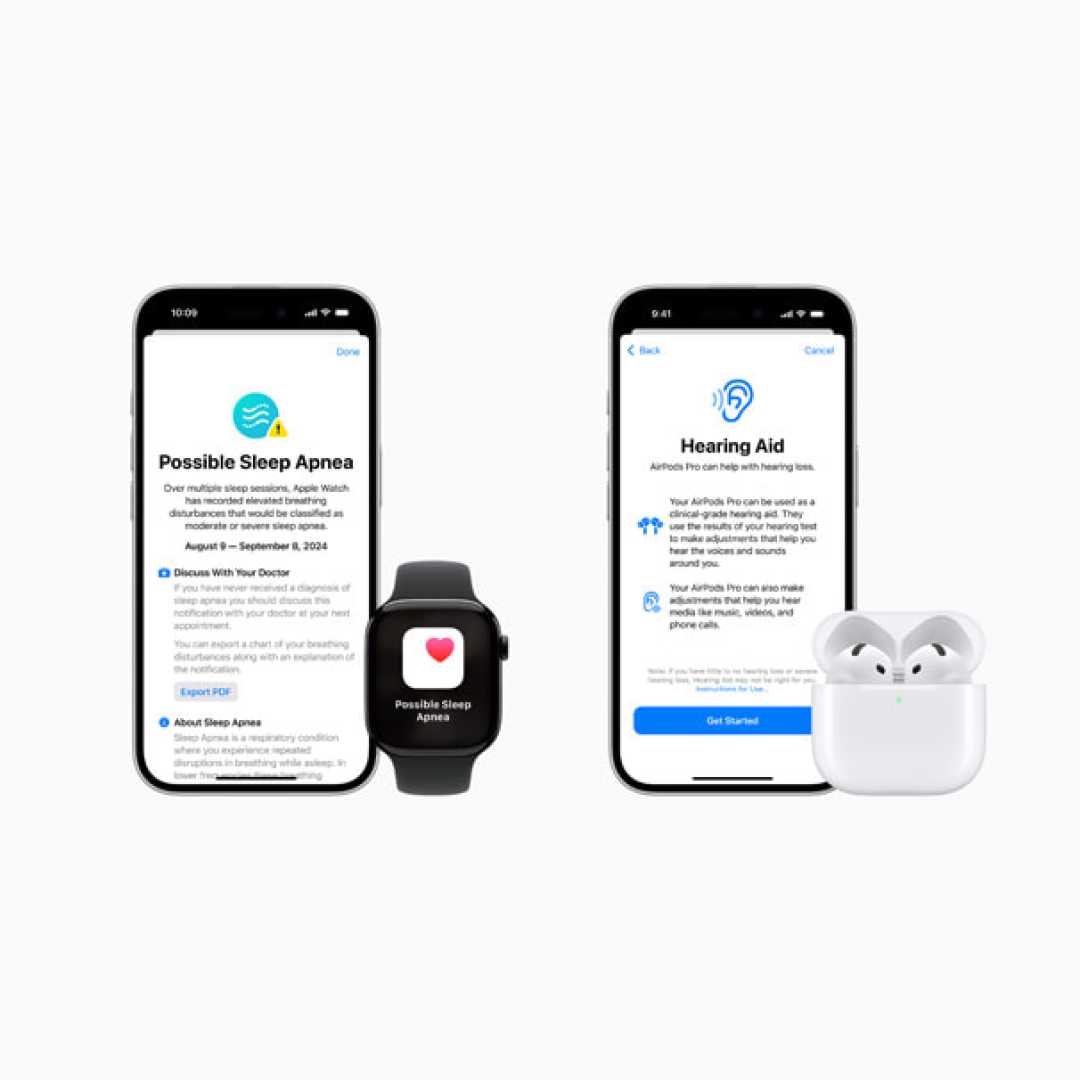
Apple has announced the rollout of new health features for its Apple Watch and AirPods Pro 2, aimed at enhancing users’ sleep and hearing health.
Among the new features is the Breathing Disturbances metric, which will be available on the Apple Watch. This innovative metric uses an accelerometer to detect small wrist movements that may indicate interruptions in normal respiratory patterns during sleep. Users will receive notifications if they exhibit consistent signs of moderate to severe sleep apnea.
The sleep apnea notifications are pending marketing authorization from the Food and Drug Administration and other global health authorities. They are expected to become available in over 150 countries and regions, including the United States, the European Union, and Japan.
Furthermore, AirPods Pro will introduce active Hearing Protection, a clinically validated Hearing Test feature, and an over-the-counter Hearing Aid function. These features are also anticipated to receive marketing authorization soon and will be accessible this fall in over 100 countries such as the United States, Germany, and Japan.
The Breathing Disturbances feature on the Apple Watch analyzes sleep patterns every 30 days and allows users to view their results over various timeframes. They may also export a report to discuss findings with healthcare providers.
Additionally, the AirPods Pro Hearing Test will utilize a standard clinical approach known as pure-tone audiometry, enabling users to perform the test at home. After completion, users will receive a summary of results stored securely in the Health app.
The new over-the-counter Hearing Aid feature allows AirPods Pro to act as a clinical-grade hearing aid for individuals with mild to moderate hearing loss. Users can adjust sound settings in real time based on their personalized hearing profile.
Apple anticipates receiving FDA clearance for these hearing assistance features shortly, which will further solidify the AirPods Pro’s status as a regulated medical device.












