Health
Legislators Warn CMS Proposals May Limit Access to Diabetes Technology
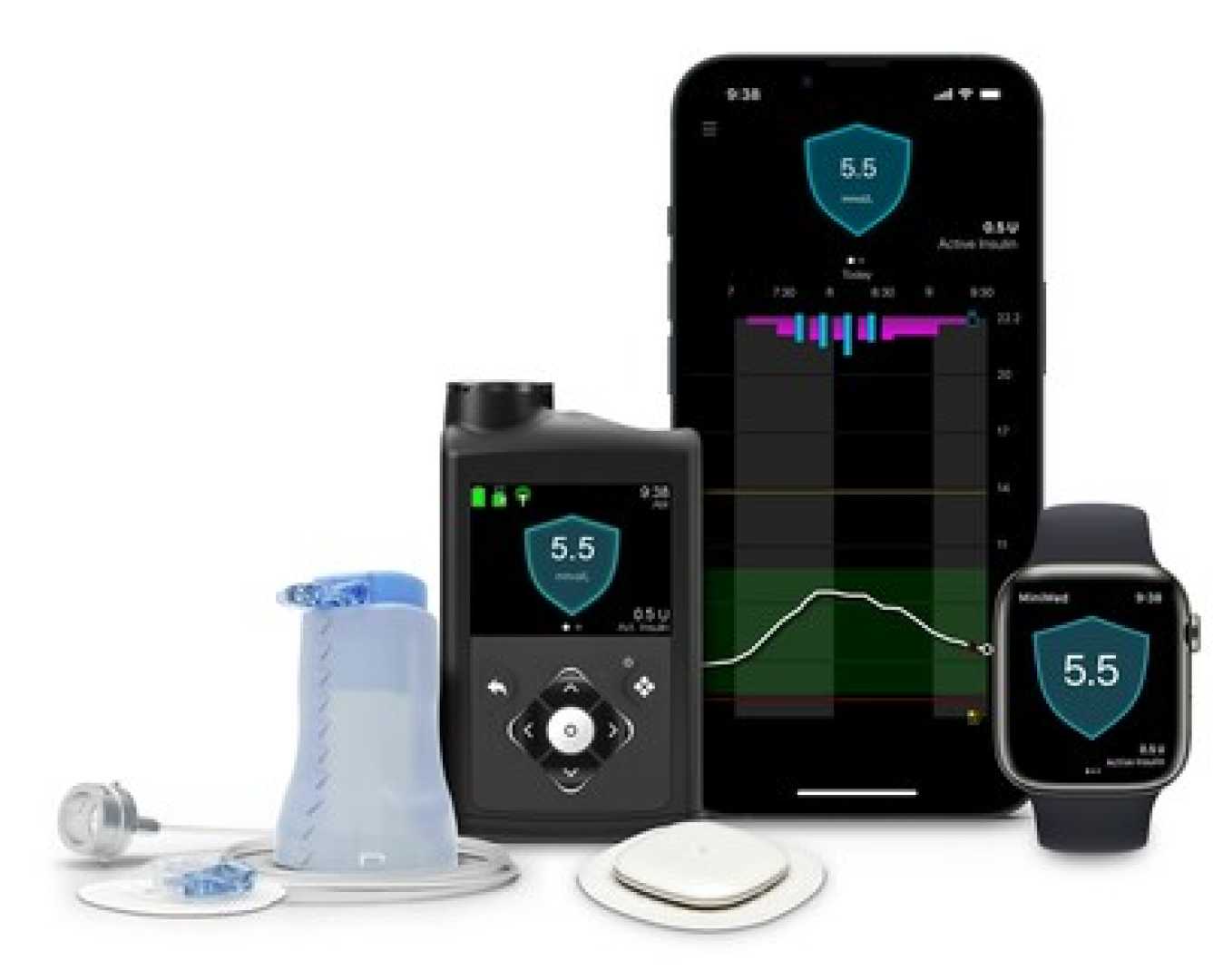
WASHINGTON, D.C. — Leaders of the diabetes caucus are expressing concerns about recent proposals from the Centers for Medicare and Medicaid Services (CMS) that they believe could limit access to essential diabetes management technologies. In a letter to CMS Administrator Mehmet Oz, Senators Jeanne Shaheen and Susan Collins, along with Representatives Diana DeGette and Gus Bilirakis, addressed their worries regarding the potential impact on beneficiaries relying on glucose monitors and insulin pumps.
This summer, diabetes technology companies, including Tandem Diabetes Care and Insulet, stated they did not expect significant adverse effects on their businesses from CMS proposals. However, the caucus leaders warned that finalizing the new rules could result in a few suppliers being responsible for providing durable insulin pumps and continuous glucose monitors (CGMs). These suppliers would also manage maintenance and software updates, shifting responsibilities away from manufacturers.
“These proposed policies could reduce choices for CGM and durable insulin pump beneficiaries,” the legislators wrote. “Suppliers might not carry all types and combinations, pushing beneficiaries towards a one-size-fits-all model.” While they support the aim of enabling beneficiaries to switch to new technologies more frequently, they outlined alternatives CMS could explore.
AdvaMed CEO Scott Whitaker voiced his concerns, emphasizing that current access to diabetes technology is already inadequate for many patients. “Any policies that undermine access are a step in the wrong direction,” Whitaker stated.
In light of these developments, experts call for careful evaluation of CMS’s initiated policies before moving forward with any competitive bidding programs for durable medical equipment.












